Humulin Production:
Recombinant DNA used to produce human
insulin
Ever since Banting and Best isolated a protein they called
"isletin" from the Islets of Langerhans from a pancreas and injected it
into diabetic dogs, insulin has been used to help diabetics live out their
lives. However, this protein had its problems. For many years,
insulin was extracted and purified from either porcine or bovine pancreases,
and this carried with it two main difficulties. The first was that
though the animal insulin was chemically similar to human insulin, there
were some differences, and these differences led to antibody attack and
inactivation as well as inflammation in many patients. Also, there
was the problem that this method of extracting insulin from animal organs
made it difficult to obtain large amounts of pure insulin.
Nowadays, it is a well known fact that DNA contains genes
which encode all the different proteins that an organism can produce.
But back then, the composition of proteins and their relation to DNA was
unknown. It wasn't until Sanger, in 1955, determined the sequence
of insulin and determined that proteins are composed of specific amino
acids linked to each other in a peptide chain. And even then, it
wasn't until 1979 that human insulin was able to be produced in large quantities.
This was done by the use of recombinant DNA.
Recombinant DNA, or rDNA, is DNA which specifically encodes
a protein. This is cut from genomic DNA by a restriction enzyme which
cuts DNA at specific sequences along the chain. These pieces are
then analyzed and the DNA needed to make the protein is extracted and purified.
Since insulin contains two polypeptide chains
linked by disulfide bonds, two pieces of DNA are extracted. These
DNA strands are then placed into two different plasmids, as shown in the
figure below.
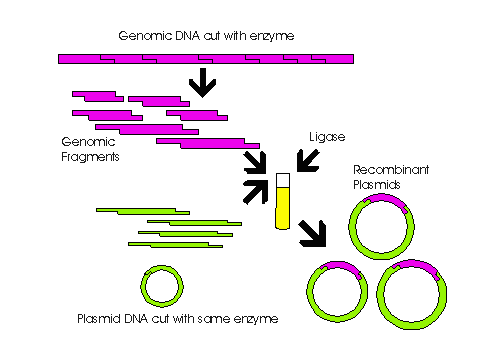
These plasmids are also cut with the same restriction
enzymes  as
the DNA,which will allow the DNA to be fixed into the plasmid by DNA ligase.
The plasmids are then incubated with a weakened strain of E. coli.
Since
only some of the bacteria will take up the plasmid,a gene encoding an enzyme
which breaks down a certain antibiotic is also included in the plasmid,
which allows bacteria with the plasmid to grow on a plate containing the
antibiotic while the other bacteria die. These bacteria are then
allowed to grow and replicate, which allows the plasmid and the insulin
gene to replicate millions of times. Then the bacteria are given
a signal to produce the protein, and insulin identical to that of humans
can be produced and purified. This insulin, which is abtly named
humulin, can then be used to treat many people with type I diabetes without
the worry of allergic reaction.
as
the DNA,which will allow the DNA to be fixed into the plasmid by DNA ligase.
The plasmids are then incubated with a weakened strain of E. coli.
Since
only some of the bacteria will take up the plasmid,a gene encoding an enzyme
which breaks down a certain antibiotic is also included in the plasmid,
which allows bacteria with the plasmid to grow on a plate containing the
antibiotic while the other bacteria die. These bacteria are then
allowed to grow and replicate, which allows the plasmid and the insulin
gene to replicate millions of times. Then the bacteria are given
a signal to produce the protein, and insulin identical to that of humans
can be produced and purified. This insulin, which is abtly named
humulin, can then be used to treat many people with type I diabetes without
the worry of allergic reaction.
For more information
on treatment of Diabetes I and II, click here.
For medications for Diabetes
type II, click here.

 as
the DNA,which will allow the DNA to be fixed into the plasmid by DNA ligase.
The plasmids are then incubated with a weakened strain of E. coli.
Since
only some of the bacteria will take up the plasmid,a gene encoding an enzyme
which breaks down a certain antibiotic is also included in the plasmid,
which allows bacteria with the plasmid to grow on a plate containing the
antibiotic while the other bacteria die. These bacteria are then
allowed to grow and replicate, which allows the plasmid and the insulin
gene to replicate millions of times. Then the bacteria are given
a signal to produce the protein, and insulin identical to that of humans
can be produced and purified. This insulin, which is abtly named
humulin, can then be used to treat many people with type I diabetes without
the worry of allergic reaction.
as
the DNA,which will allow the DNA to be fixed into the plasmid by DNA ligase.
The plasmids are then incubated with a weakened strain of E. coli.
Since
only some of the bacteria will take up the plasmid,a gene encoding an enzyme
which breaks down a certain antibiotic is also included in the plasmid,
which allows bacteria with the plasmid to grow on a plate containing the
antibiotic while the other bacteria die. These bacteria are then
allowed to grow and replicate, which allows the plasmid and the insulin
gene to replicate millions of times. Then the bacteria are given
a signal to produce the protein, and insulin identical to that of humans
can be produced and purified. This insulin, which is abtly named
humulin, can then be used to treat many people with type I diabetes without
the worry of allergic reaction.