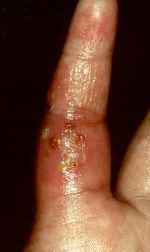
 Staphylococcus
aureus causes a variety of suppurative (pus-forming) infections and toxinoses
in humans. It causes superficial skin lesions such as boils, styes and
furuncles; more serious infections such as pneumonia, meningitis, and urinary
tract infections. S. aureus is a major cause of hospital acquired
(nosocomial) infection of surgical wounds and infections associated with
indwelling medical devices. S. aureus causes food poisoning and toxic shock
syndrome. The toxins mostly responsible for causing these infections and
diseases in humans are the superantigens and alpha-toxins. The alpha-toxins
oligomerize to form pores in the host cellular membrane, allowing cellular
contents to leak into the extracellular matrix. The superantigens,
consisting of enterotoxins and the toxic shock syndrome toxin, are responsible
for S. aureus-related food poisoning and toxic shock syndrome, respectively.
(See Figures 1 for how alpha-toxins and superantigens work)
Staphylococcus
aureus causes a variety of suppurative (pus-forming) infections and toxinoses
in humans. It causes superficial skin lesions such as boils, styes and
furuncles; more serious infections such as pneumonia, meningitis, and urinary
tract infections. S. aureus is a major cause of hospital acquired
(nosocomial) infection of surgical wounds and infections associated with
indwelling medical devices. S. aureus causes food poisoning and toxic shock
syndrome. The toxins mostly responsible for causing these infections and
diseases in humans are the superantigens and alpha-toxins. The alpha-toxins
oligomerize to form pores in the host cellular membrane, allowing cellular
contents to leak into the extracellular matrix. The superantigens,
consisting of enterotoxins and the toxic shock syndrome toxin, are responsible
for S. aureus-related food poisoning and toxic shock syndrome, respectively.
(See Figures 1 for how alpha-toxins and superantigens work)
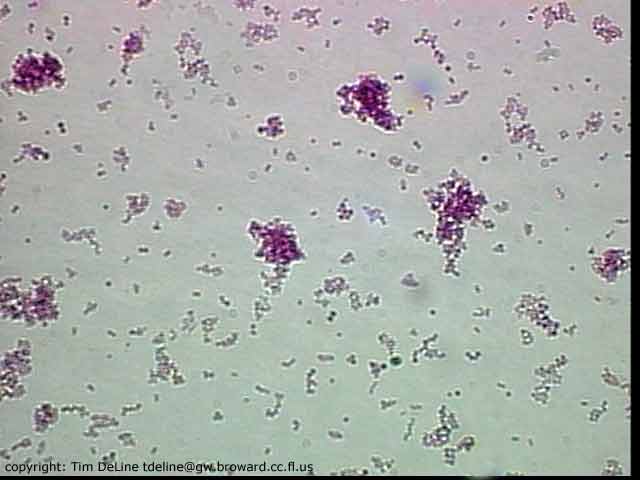 Staphylococcus
aureus is a Gram-positive, spherical bacteria that occur in microscopic
clusters resembling grapes. S. aureus colonizes mainly the nasal passages
of humans, but it may be found in most other anatomical locations.
Staphylococcus
aureus is a Gram-positive, spherical bacteria that occur in microscopic
clusters resembling grapes. S. aureus colonizes mainly the nasal passages
of humans, but it may be found in most other anatomical locations.