An Insight into Insulin and the Role its Plays in Diabetes Mellitus
Insulin was the first hormone identified (late 1920's) which won the doctor and medical student who discovered it the Nobel Prize (Banting and Best). They discovered insulin by tying a string around the pancreatic duct of several dogs. When they examined the pancreases of these dogs several weeks later, all of the pancreas digestive cells were gone (died and were absorbed by the immune system) and the only thing left was thousands of pancreatic islets. They then isolated the protein from these islets and behold, they discovered insulin.
Insulin is one of the most important hormones, carrying messages that describe the amount of sugar that is available from moment to moment in the blood. Insulin is made in the pancreas and added to the blood after meals when sugar levels are high. This signal then spreads throughout the body, to the liver, muscles and fat cells. Insulin tells these organs to take glucose out of the blood and store it, in the form of glycogen or fat. Your body stores very little glycogen at any one time, all the glycogen stored in your liver and muscle wouldn’t last you through one active day. Once you fill up your glycogen stores that sugar is stored as saturated fat. Once in the blood, insulin controls glucose homeostasis by stimulating the uptake of glucose into skeletal muscle and, to a lesser extent, into liver and adipose tissue. The enzymes involved in the insulin-regulated processes of glucose metabolism appear to be regulated by (de)phosphorylation of serine and/or threonine residues. All known actions of insulin are initiated at the plasma membrane by insulin receptors responding to ligand binding.
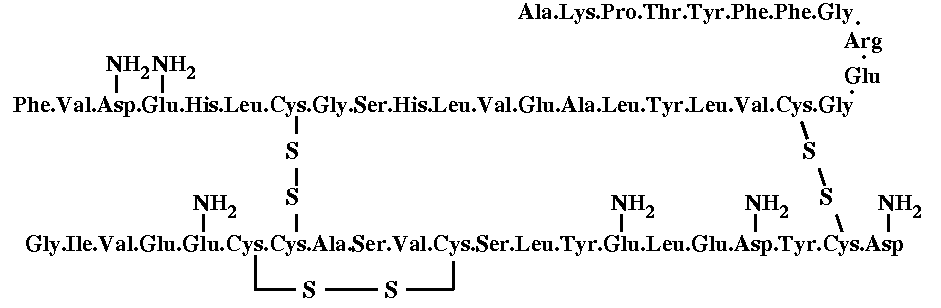
Human insulin consists of 51 amino acids, divided into two chains, commonly labelled A and B, with 21 and 30 amino acids respectively. The chains are linked by three disulfide bridges, two forming interchain cystines at A7-B7 and A20-B19, and one forming an intrachain cystine at A6-A11. A piece of antiparallel b-sheet is formed upon dimerisation: residues B23 to B28 of one monomer lie antiparallel to the same stretch in the other monomer. There are two very small a-helices in the A chain, and a three turn a-helix running from residues B9 to B19 is found in every insulin structure known so far. The complete sequence of insulin is the following:

The molecule
is complexed with two zinc atoms. In the pancreas, zinc is incorporated
into the b granule to form the nucleus around which insulin deposits in
a hexameric array, which probably gives the b-cell granule its crystalline
look. It is certrain that in most creatures where zinc is present in the
pancreas, the insulin molecules must be stored in hexamers to be released
through the cell membrane as insulin secretion is stimulated.
This is a view of a insulin dimer.
Fortunately,
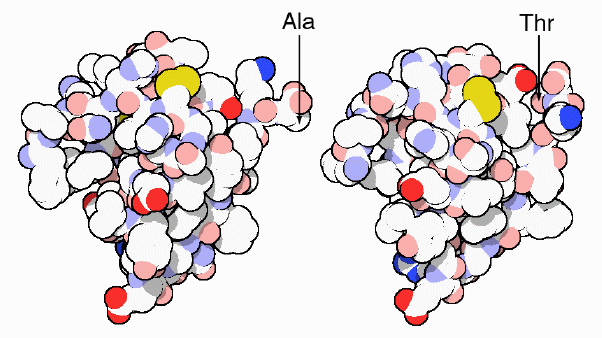
insulin from pigs (at left) differs from human insulin (at right) by only
one amino acid: a threonine at the end of the chain in human insulin is
replaced by alanine in pig insulin. Insulin from cows is also very similar,
differing in only three positions. Because of their similarity, these forms
of insulin are also recognized by our own cells and may be used in therapy.
Links-
Introduction to Diabetes Mellitus: Type I and Type II Diabetes Mellitus
The Insulin Receptor and Diabetes Mellitus
Diagnosis and Treatment of Diabetes Mellitus
Insulin Therapy Used for Type I Diabetes Mellitus