Cholera Toxin
[Sources and Historical Background] [The Culprit Protein] [At the Molecular Level] [Effects] [Treatment] [References and External Links]
Sources and Historical Background
Image courtesy of NSF
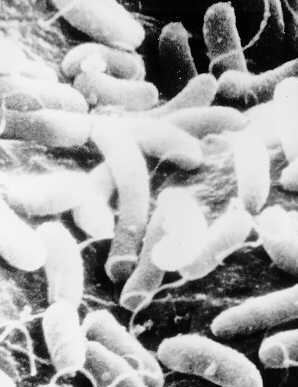 Cholera toxin is produced by the bacterium Vibrio cholerae, shown at left. It is a gram negative curved rod (hence vibrio) with a single polar flagellum. This flagellum allows the bacterium to be motile, thus it can "swim" against a current. The flagellum can also be a tool of attachment to host tissues in some cases. It is a facultative anaerobe, meaning its metabolism is adapted to function in both aerobic and anaerobic conditions. It is quite common and is often found on surface waters, both fresh and saltwater.
Cholera toxin is produced by the bacterium Vibrio cholerae, shown at left. It is a gram negative curved rod (hence vibrio) with a single polar flagellum. This flagellum allows the bacterium to be motile, thus it can "swim" against a current. The flagellum can also be a tool of attachment to host tissues in some cases. It is a facultative anaerobe, meaning its metabolism is adapted to function in both aerobic and anaerobic conditions. It is quite common and is often found on surface waters, both fresh and saltwater.
Proper water filtration systems and effective sewage treatment facilities ensure that V. cholerae does not cause problems with humans. It is for that reason that Cholera cases are few in the United States and most developed nations. Most cases that do occur in developed nations can often be traced back to foreign travel to underdeveloped nations with an unclean drinking water supply. However, without proper sanitation, Cholera can easily affect humans. If left untreated, it has a fatality rate of up to 50%. However easy means of treatment, explained later, lower the fatality rate to 1% in adults and 2% in children.
Epidemic cholera, as it is often called, has been affecting humans at epidemic levels for centuries. One particularly interesting case is the Broad Street Pump Outbreak in London, England. In the summer of 1854, cholera epidemics had been sweeping across England. More than 10,000 in London alone were dead. In August, a peculiar pattern emerged. Within 3 days 127 people died all in one area. The dead had nothing in common-neither wealth nor living conditions nor ethnic heritage seemed to have any influence on virulence of the disease. The only thing these people had in common was that they drew their water from the same place, the Broad Street Pump. By September the number of dead soared to 500. Dr. John Snow, a surgeon in the area, conducted exhaustive interviews and examined the water under a microscope. His results were consistent with his theory that the disease was spread through contaminated water. As an experiment, he asked the officials to remove the handle to the pump. Within days, the death rate dropped.
The Culprit Protein
Chime Image Courtesy of Brookhaven Protein Database
 Cholera toxin is produced by the bacterium Vibrio cholerae, shown at left. It is a gram negative curved rod (hence vibrio) with a single polar flagellum. This flagellum allows the bacterium to be motile, thus it can "swim" against a current. The flagellum can also be a tool of attachment to host tissues in some cases. It is a facultative anaerobe, meaning its metabolism is adapted to function in both aerobic and anaerobic conditions. It is quite common and is often found on surface waters, both fresh and saltwater.
Cholera toxin is produced by the bacterium Vibrio cholerae, shown at left. It is a gram negative curved rod (hence vibrio) with a single polar flagellum. This flagellum allows the bacterium to be motile, thus it can "swim" against a current. The flagellum can also be a tool of attachment to host tissues in some cases. It is a facultative anaerobe, meaning its metabolism is adapted to function in both aerobic and anaerobic conditions. It is quite common and is often found on surface waters, both fresh and saltwater.