Combination Therapy with Allosteric and Competitive Inhibitors of the Abl Kinase
Kirk D. Wyatt
Chronic myelogenous leukemia (CML) is a form of cancer that affects white blood cells. CML involves deregulation of the protein tyrosine kinase Abl (shown in the left pane; PDB ID: 3K5V).
A common treatment for CML is the drug imatinib, which is an ATP-competitive inhibitor of Abl. Imatinib is of noteworthy significance in the pharmaceutical industry because it was the first first protein tyrosine kinase inhibitor to be approved by the FDA for human use. Others are currently in development.
Click here to see imatinib bound to Abl.
One factor limiting the drug’s efficacy is the emergence of imatinib-resistant mutants of the protein. Other ATP-competitive inhibitors (i.e., nilotinib and dasatinib) have been developed to inhibit some of the mutant forms of the kinase which have emerged; however, these drugs are largely powerless against certain mutants, such as those expressing the so-called "gatekeeper" T315I mutation. The T315 residue lies at the heart of the ATP binding site, where imatinib binds (click here to show residue T315). Given the important role of structure in the regulation of enzyme function, the emergence of mutations at the active site poses as a significant hurdle for inhibition.
Another significant landmark on the protein, aside from the ATP binding cleft (targeted by imatinib, nilotinib and dasatinib) is the myristate binding pocket. Abl binds to myristate in the process of cell membrane integration. Click here, to show GNF-2, a myristate mimic, bound in the myristate binding pocket of Abl. Although a few water-mediated hydrogen bonds (not shown) are involved, the binding interaction is largely hydrophobic, as we would expect from a binding pocket which naturally binds myristate, a nonpolar fatty acid. Clicking here illustrates the largely hydrophobic nature of the residues in the vicinity of the myristate binding pocket. The interior of the binding pocket is populated by hydrophobic residues, and non-hydrophobic residues are concentrated on the exterior protein surface.
Structure-activity relationships for the binding of GNF-2 to the myristate binding pocket were eventually elucidated through the synthesis and evaluation of over 200 GNF-2 analogs. The key findings of these studies are shown below in Figure 1.
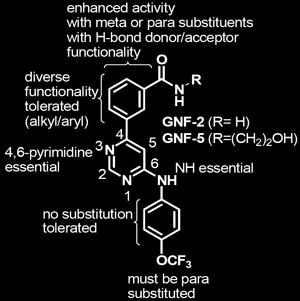
|
| Figure 1. Structure-activity relationships (SAR) for GNF-2 & 5. Structures of GNF-2 and GNF-5 highlighting features important for Bcr-Abl inhibitory activity. |
Although GNF-2 binds suitably to the protein in vitro, it has poor pharmacokinetic properties in vivo. The investigators identified the analog GNF-5, based on the structure-activity relationships shown in Figure 1, which has similar binding properties as GNF-2 and more favorable pharmacokinetics. Further studies on myristate analogs, therefore, focused on GNF-5.
So, why do we care about myristate mimics?
It turns out that GNF-2 is an inhibitor of Bcr-Abl (IC50 = 0.15 µM). Moreover, when mutant proteins were studied, only those which involved mutations at the myristate binding site were resistant to GNF-2 inhibition. Mutants that had been modified at the ATP binding site were still susceptible to inhibition by GNF-2. The mechanism of this non-ATP competitive inhibitor, then was ostensibly allosteric; however, the exact mechanism by which allosteric inhibition was achieved was unclear.
Through the use of hydrogen-exchange mass spectrometry, it was found that binding of GNF-5 in the myristate binding pocket induces conformational changes in two locations on the protein. The most significant of these locations happens to be within the ATP binding site. Click here to see the portions of the protein that undergo significant (i.e., 1.0 Da or more) conformational changes upon binding to GNF-5. Feel free to twirl the protein structure around to get a better picture of the areas of the protein that change conformation.
The finding that binding of GNF-5 to the myristate binding site of the protein causes a significant change in conformation of the ATP binding site provides convincing evidence for an alloseric mechanism of inhibition. It is understood that since GNF-5 alters the conformation of the ATP binding site remotely, its efficacy is unaffected by mutations that occur within the ATP binding site (such as the T315I mutation).
Further in vivo studies on mice showed that combination treatment with GNF-5 and nilotinib, an ATP-competitive inhibitor of Abl, gave increased benefit over either individual treatment alone (P = 0.002) where the mice expressed Abl that exhibited the “gatekeeper” T315I mutation within the ATP binding pocket. Thus, combination treatment with myristate- and ATP-binding site inhibitors may provide a novel treatment modality to combat drug-resistant mutants of Abl.
Reference: (PDB ID: 3K5V) Zhang, J.; et al. Nature 2010, 463, 501. (Article Link)
Navigation: < Last Page | Main Page | Next Page >